Generation of monocyte-derived DC under GMP conditions
Laboratory protocol
Monocyte source: leukapheresis product (rarely: buffy coats of healthy blood donors, research use only) => gives high yield of monocytes (15-25 x 10e8)
Monocyte isolation: affinity purification (CliniMacs) or counter-flow centrifugation (Elutra) => good recovery, high purity, high viability
Culture: in culture bags (Teflon Bags, Cell Genix) in serum free medium (CellGro from Cell Genix) => no contact-mediated activation, no interference by serum proteins
For research use (sometimes) generation of adherent DC by plating monocytes on plastic.
Cytokines: clinical grade GM-CSF (Leukine, Berlex) and GMP-grade IL-4 (Cell Genix)
Maturation (usually day 5): clinical grade TNF-? (Cell Genix) or poly I:C (research use only) for 48 hours
Antigen loading on immature DC: electroporation, viral transduction, pulsing with peptides/cell lysate/apoptotic bodies
Total recovery of usable immature/mature DC: 40/30 % +/- 10%
Application: DC vaccines, generation of CTLs (for adoptive transfer of T cells or research use), immunomonitoring (targets in read-outs for T cell adoptive transfer therapy)
-
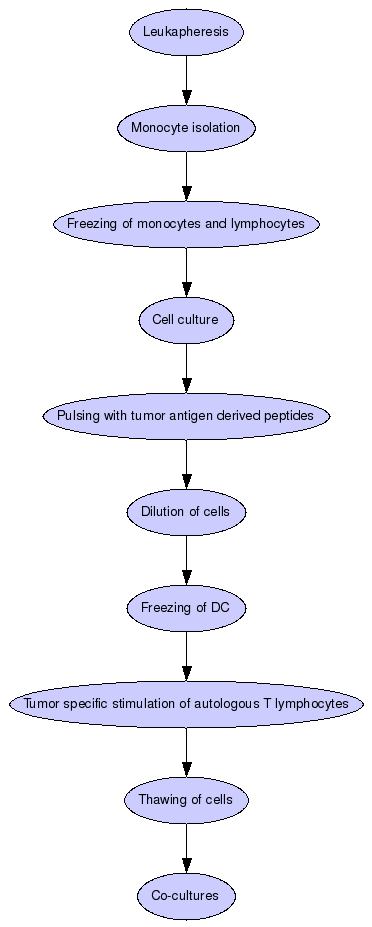
Leukapheresis
A HLA-A2 positive healthy donor undergoes leukapheresis.
-
Monocyte isolation
Cells are subsequently separated into monocytes and lymphocytes by elutriation in a closed system (Elutra).
The two most monocyte-rich fractions collected contained > 80% monocytes, the fraction richest in lymphocytes contained > 90% lymphocytes and viability in both fractions was > 95%.
-
Freezing of monocytes and lymphocytes
The majority of monocytes (1 x 10e9) and all lymphocytes (6,4 x 10e9) are frozen in 90% A-plasma and 10% GMP-grade DMSO.
-
Cell culture
2 x 200 x 10e6 monocytes are transferred into culture bags by sterile welding and cultured for 5 days at a density of 2.5-5 x 10e6 cells/mL in serum free medium (CellGro, Cell Genix) in the presence of 100 ng/mL GM-CSF (Leukine, Berlex) and 20 ng/mL IL-4 (Cell Genix). After 2 days one volume fresh medium containing 2x concentrated cytokines is added.
-
Pulsing with tumor antigen derived peptides
On day 5 cells are counted and pulsed with tumor antigen derived peptides (one per culture) at a concentration of 5 µg/mL in approximately 5 mL and at a cell density of 20 x 10e6/mL for 1 h.
-
Dilution of cells
After peptide pulsation cells are diluted in medium containing 20 ng/mL TNF-?, plus IL-4 and GM-CSF as stated above.
-
Freezing of DC
On day 7 of culture 35/40 x 10e6 mature peptide pulsed DC are frozen at 5 x 10e6 cells per vial in freezing medium as above.
-
Tumor specific stimulation of autologous T lymphocytes
The frozen MoDC are subsequently used for tumor peptide specific stimulation of autologous T lymphocytes at a ratio of 1 DC per 10 T cells.
Co-cultures led to antigen specific, IFN-? secreting T cells after 1 restimulation at a DC:T cell ratio of 1:20, as evaluated by IFN- ? Elispot, showing that the generated DC have good antigen presenting and co-stimulatory capacity.
-
Thawing of cells
After thawing cells showed good viability (>90%) and the recovery was approximately 80%.
- biomaterial type
- dendritic cell,
- monocyte,
- Granulocyte macrophage colony-stimulating factor,
- Interleukin-4,
- Polyinosinic acid:polycytidylic acid
created over 17 years ago
(2 March 2009)
last modified over 14 years ago
(28 September 2011)
[ RDF  ]
[ RelFinder
]
[ RelFinder  ]
]
